Abstract
Introduction: Pyruvate kinase deficiency (PK def) is an inherited disorder that affects red blood cells metabolism and causes a wide range of clinical manifestation from fetal anemia, severe neonatal jaundice, and chronic transfusion dependent anemia to fully compensated hemolytic anemia. This condition is rarely reported in Southeast Asian population due to a lack of knowledge and recognition by clinicians and unavailability of laboratory for confirmation. The majority of patients with PK def result from mutations of the PKLR genes encoding this enzyme. Our group has described for the first time (Viprakasit V et al., Blood 2014) that mutations in trans of an erythroid specific transcription factor; KLF1 that regulate PKLR expression, could result in clinical presentation mimic that of classical PK def. Moreover, α and β globin mutations are highly prevalent in our region (up to 40% carrier rate) but their roles as a genetic modifier in PK def remain unknown. In addition, mutations in KLF1 seems to be highly prevalent in our population (14.6 in 1000, n= 2464 chr.), it is of interest to determine the epistatic role of α and β globin, PKLR and KLF1 mutations in patients with classical presentation of PK def.
Objectives: To characterize α and β globin, PKLR and KLF 1 mutations and correlate these with clinical presentation and outcome(s) of them in patients with PK def and their family members.
Methods: After obtaining informed consent, 9 index PK def patients with their available family members were evaluated for PKLR and KLF1 mutations by direct genomic sequencing as previously described. Hematological parameters and hemoglobin analyses were performed using standard techniques. DNA analysis for all common α and β globin mutations leading to thalassemia and hemoglobinopathies found in this region was performed using our standard protocol.
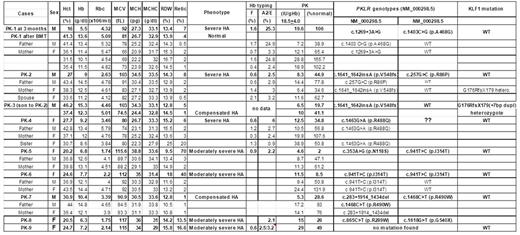
Results: We successfully identified PKLR mutations in 7 out of 9 index patients as shown in Table 1. All patients were diagnosed with clinical PK def following several measurement of biochemical activity. We found 11 different mutations in 6 compound heterozygotes (5 of two nucleotide mutations and one with 1 missense and a large PKLR deletion) and 1 homozygote. One mutation (c.941T>C =p.I314T) seemed to be recurrent since it was found in two families; one homozygous and one compounded with N118S. We failed to identified PKLR and KLF1 in only one patient (PK-9) suggesting that this patient might have a novel molecular mechanism that remains to be found. We found only one family with globin abnormality (Hb E trait in PK-1) without any deleterious effect on patient's clinical severity. Moreover, most of PK patients (except one) have no mutation in KLF1 genes. Interestingly, the PK-3 patient (who is an offspring of the PK-2) was subsequently found to be double heterozygous of PKLR (p.V548fs) and KLF1 (G176RfsX179 (+7bp)) mutations. This patient was previously diagnosed with PK def at birth when he had serious hemolytic anemia (Hb 10 g/dL, haptoglobin < 10 IU/L) with hyperbilirubinemia requiring phototherapy, based on a very low PK enzyme activity (6.5 IU/g Hb, 20% of normal level) and the PK level from his mother was also low (11 IU/g Hb). Since then, he had an uneventful history for 6 years, except mild anemia from iron deficiency anemia during infant period. Physical examination showed no hepatosplenomegaly. Currently his baseline Hb levels were around 12-12.5 g/dL with normal reticulocyte count (1%) and hypochromic microcytosis. There is no globin abnormality in this family. The PK enzyme activity at 6-year-of age showed an improved PK activity (41% of normal activity) without further hemolysis. Our finding showed for the first time a possible interaction between PKLR and KLF1 mutations in man and this digenic inheritance seems to cause a more serious phenotype than each of these carriers alone. However, since the clinical presentation of our index case (PK-3) appeared to be apparent only at the early age, this might suggest the possible impact of KLF1 mutations that were more serious at the early age (similar to those of patients with two KLF1 mutations; KLF1 disease) and might be further compensated by other hitherto mechanisms during erythroid development.
Conclusions: We found no effects of globin mutations on the clinical phenotypes of patients with classical PK def. However, a mutation in KLF1 could deteriorate clinical presentations even in a carrier of PKLR mutation at the early age.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal